Let’s try this for starters: This is such an amazing project!!! It is environmental molecular science at its best, it bridges the length scale of a molecular bond, which is ridiculously short, with the ocean and the atmosphere, which are ridiculously big, and it brings the real world into a chemist’s lab! You couldn’t have done this kind of stuff ten years ago, and it is beautiful to see it all unfold right in front of our eyes.
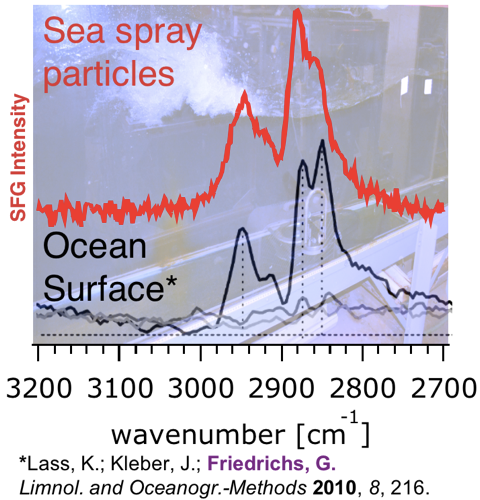
Our first spectra of organic molecules on sea spray produced by the breaking waves in the Scripps wave tank (top) and the spectra of organic molecules on the top surface of the Baltic sea (bottom) seem to point us into the right direction.
We are physical chemists from Northwestern University in Evanston, which is the first suburb just north of Chicago. It’s not nearly as nice in San Diego as it is in Chicago during the month of November, but then again … what? No, of course we enjoy it a lot! Franz Geiger is blogging – he is the Irving M. Klotz professor of physical chemistry at NU. What’s physical chemistry? well, it’s physics – and chemistry – combined! There’s even a journal named after it – go check it out at http://pubs.acs.org/journal/jpcafh. Carly Ebben is a third-year PhD student in the Geiger group who is also a National Science Foundation Graduate Student Fellow. She participated in a really cool large-scale study in Southern Finland just last year, and together with samples collected during an equally impressive study in the central Amazon, Carly is now managing the sample acquisition, data collection and analysis, and paper writing for three important field studies, which is the focus of her PhD thesis.
Here at the tank, we are studying the chemical composition of sea spray particle surfaces – not the bulk, which contains many, many molecules – but the surface, which houses much fewer molecules. Try it out on a sheet of paper – sketch a cube and fill it with small circles, then count how many are at the surface vs. the interior. In fact, there are so few molecules on the surface of a particle compared to the particle interior that one needs some pretty nifty tools to detect them – that’s what we bring to the CAICE. A second motivation for our studies is that there is a high likelihood that our methods can distinguish surface molecules on sea spray particles prepared in the tank when bugs are absent and when bugs are present – like a signature of life, if you will. This is how it works: We all know that living things contain DNA and proteins, and that the walls of cells are made of phospholipids and sugars. These compounds have a particular handedness, which allows for key molecular processes to occur in the biological machinery that we call ‘life’. A core concept in these biochemical processes is that of molecular recognition – and the handedness we just mentioned plays a key role in it: Imagine the handshake between two people – it typically involves the right hand of one person shaking another person’s right hand – the same happens in molecular recognition. If you were to shake somebody’s right hand by extending your left hand, it’ll be really awkward to say the least – the same is true for proteins and DNA, for which handedness is a key requisite for proper function. But for us, handedness is also an intrinsic marker for the presence of biological material on the particles that are produced at the wave tank, and that is what we are here to study these two weeks. This is a tough problem, because we look for the footprints of life without having to destroy the particles. To do so, we combine particle sampling at the tank with ultrafast laser spectroscopy at Northwestern University – this approach has already allowed us to establish that the particles from the wave tank have organic species on them, and we didn’t have to destroy the particles to learn that.
How do we do it? We began work at the tank one week ago by installing sampling systems that allow us to collect sea spray particles for a certain amount of time and in certain size ranges on Teflon filters – and yes, even though it’s common sense that ‘nothing sticks to Teflon (hence the famous pan)’, the samplers we use can do the job because of some pretty cool particle collection physics. Nevertheless, there are very few particles on the filters, and not many tools can be used to detect them. Our lasers can do it, though, because they are quite special: we can adjust their energies – i.e. colors – to match those of the molecules we are looking for, and the pulses our laser produce are just one tenth of one millionth of a millionth of a second short, which means we don’t burn up our samples like Han Solo’s blaster did when he sat across Greedo, Jabba’s repo man. We detect the signals with a supersensitive camera chip that’s cooled to the surface temperature of the dark side of the moon – because it’s so cold, unlike the camera in your cell phone, ours picks up really little noise, so it’s perfect for the job (but also much more expensive). Still, the samples we are studying only produce a few photons each minute, and so we need to work carefully.
So, what do we learn? We learn that while water is of course important in ocean spray, the surfaces of the sea spray particles contain organic molecules, and it is those molecules that interact with the external world and that are important for the climate system. The graphic on the left shows the spectral signature of those organic molecules on particles collected during the crashing of breaking waves inside the wave channel, and how that is in qualitative agreement with what professor Gernot Friedrichs, a colleague of Franz’ in Kiel, Germany, published recently when he applied a similar laser method to the sea surface microlayer he collected on the other side of the planet – the Baltic Sea in Northeastern Europe! Now, here at the wave tank in Southern California we looked at the organic molecules on the surfaces of the sea spray particles, and the Baltic sample was collected by skimming the ocean surface using a boat miles offshore, but doesn’t the good agreement between the data suggest that the organic molecules on the ocean surface are similar to the organic molecules we see on the sea spray? And wouldn’t that suggest that when phytoplankton is present in the ocean, the biomolecules associated with the bugs could be associated with the sea spray particles? And doesn’t that imply that there may be a possibility for a biosphere-atmosphere feedback cycle that would be awesome to understand if we want to understand the complexity of the climate system? That’s where our part of the CAICE project is going, and that is what we’ll be looking for when the wave tank is filled with critters tomorrow!
Franz Geiger, Irving M. Klotz Professor of Chemistry & Associate Chair, Department of Chemistry, Northwestern University




