As a chemist, engaged in the study of climate, I often ask myself and my colleagues:
At what point do chemical mechanisms overtake biological and physical process and assume control over environmental systems.
To answer this question and ultimately study chemical mechanisms in the environment, the biological and physical landscapes need to not only be defined, but controlled. There is arguably no place in nature that is more chemically, biologically, and physically complex than the surface of the ocean. This microcosm, is physically separated from the bulk ocean due to the suppression of turbulence at the interface, while being a reservoir for the various end products of biological mechanisms in the surface ocean. As such, a unique chemical environment is established in the top 100s of nanometers of the ocean surface; one that is heavily enriched in insoluble organic molecules and where transport is limited by molecular diffusion. This interface displays extreme spatio-temporal heterogeneity around Earth’s planet and represents a whopping 71% of the surface of the Earth. As part of IMPACTS 2014, CAICE is generating this mesocosm in a controlled fashion so that we can study chemical processes.
Again, as a chemist engaged in climate, my group and I set out to ask chemically specific, but relevant questions, such as:
What is the net loss rate of trace gases such as dinitrogen pentoxide, a critical ingredient in photochemical ozone production, to the ocean surface?
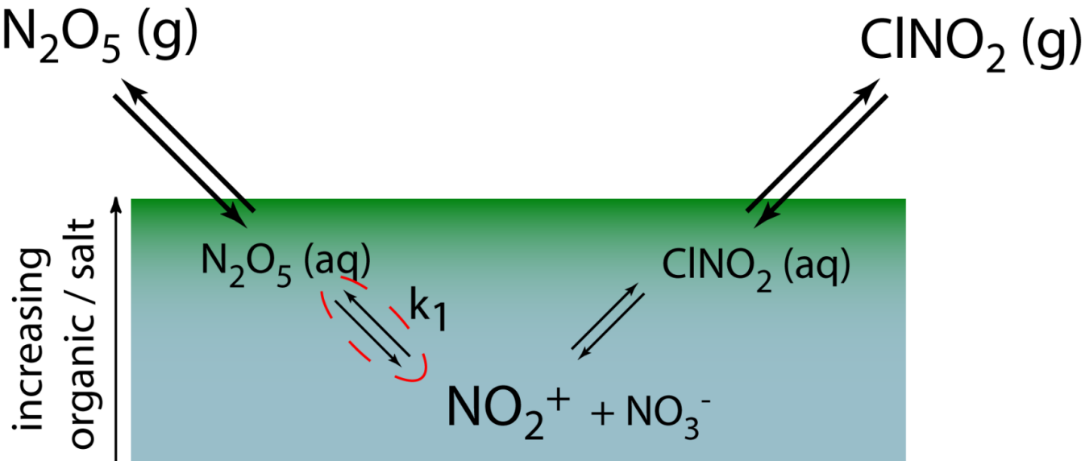
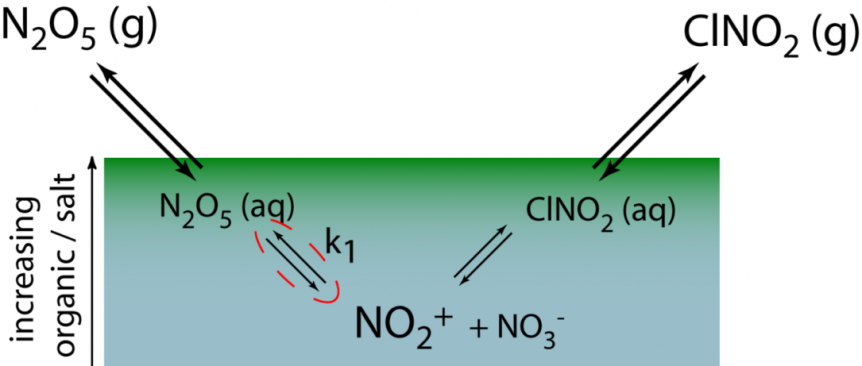
Schematic depiction of the reactive uptake of dinitrogen pentoxide (N2O5) at the surface of the ocean (top 100 nm)
Mechanistically, N2O5 readily hydrolyzes upon being accommodated into a liquid. This process is fast. So fast that N2O5 is fully dissociated into NO2+ and NO3– within tens of nanometers of the air-sea interface, well within the rich organic film of the sea-surface microlayer. As a result, the fate of NO2+ and the potential for making chlorinated products such as ClNO2 is set by the concentration and speciation of organic layer. During IMPACT 2014, we are collecting surface sea water throughout the course of the bloom in the wave channel. We then take these samples back to the laboratory where we flow trace N2O5 over the surface and measure the yield of ClNO2 from the surface waters. The ClNO2 yield provides a direct probe for the fate of NO2+ in the SSML and the ability of surface waters to activate chloride in seawater into photolabile chlorine compounds that can eventual alter the composition of the atmosphere. The results learned here will serve as a bridge between experiments done throughout CAICE using molecular beams of N2O5 as well as studies of single particle chemical transformation following trace gas uptake. It will be an exciting year!
Timothy Bertram, Associate Director of CAICE, Department of Chemistry and Biochemistry, University of California, San Diego