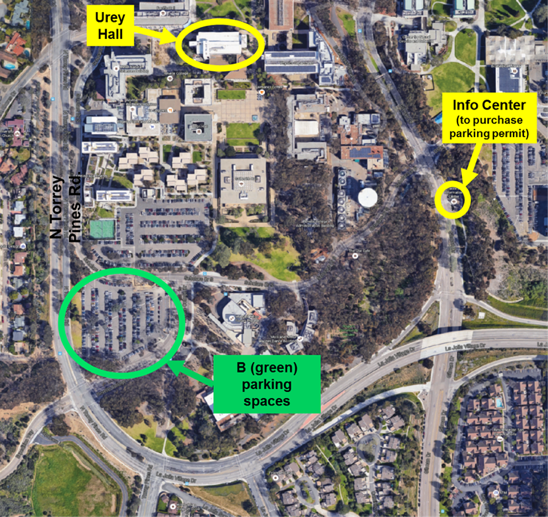
Environmental and Complex Analysis Laboratory (ECAL)
Development of novel analytical methodologies to support researchers' needs to measure within complex chemical systems.
Welcome to the home of the Environmental and Complex Analysis Laboratory. New in 2015, leveraged on the partnerships between CAICE, ThermoFisher Scientific and active support from the University of California San Diego, the facility embodies the continual development of novel analytical methodologies to support researchers continually faced with the need to measure within complex chemical systems.
ECAL Director: Dr. Neal Arakawa
narakawa@ucsd.edu
(858) 822-3330

Lab Overview
ECAL is a state-of-the-art analytical facility that houses cutting-edge chromatography and mass spectrometry instrumentation, and serves as a platform to develop innovative strategies for performing sensitive, accurate and precise analysis of complex matrices. The laboratory’s expertise and resources are available to researchers across the campus of UC San Diego and to researchers across the globe. As no two samples are alike, complex samples typically require innovative approaches for their analysis. Comprehensive compositional analysis of complex and environmental samples can be implemented through the following state-of-the-art analytical systems within the ECAL facility:
- Ultra-high resolution hybrid linear ion trap-Orbitrap mass spectrometer
- Gas chromatograph coupled to a triple quadrupole MS
- 2-D and 1-D ultra-performance liquid chromatography systems with UV/Vis and electrochemical detection
- Nano-liquid chromatography systems
- Inductively-coupled plasma quadrupole mass spectrometer
- Ion chromatograph with conductivity detector
All of the UPLC systems are capable of being hyphenated to the LIT-Orbitrap MS system. Furthermore, other hyphenated and unique techniques that are available (but are not limited to) include: UPLC-Orbitrap-ICPMS, IC-ICPMS, single-particle-ICP-MS (spICP-MS), SPME-GC-MS, and thermal desorption/pyrolysis-GC-MS. For inquiries about these unique approaches and the possibility for others not listed here, please contact the ECAL staff. Routine mass spectrometry applications include organic, organometallic, inorganic and elemental analyses. For organic and organometallic compounds, available measurements include high accuracy molecular weight determination, structural characterization through hyphenated MS techniques (including CID, HCD and ETD), as well as quantitative analysis through both GC-MS and UPLC-MS. For inorganic and elemental analysis, available measurements include quantitation of inorganic ions and individual elements, speciation of individual elements (e.g., simultaneous quantitation of Fe2+ and Fe3+), as well as the characterization of metallic nanoparticles.
Lab Overview
ECAL is a state-of-the-art analytical facility that houses cutting-edge chromatography and mass spectrometry instrumentation, and serves as a platform to develop innovative strategies for performing sensitive, accurate and precise analysis of complex matrices. The laboratory’s expertise and resources are available to researchers across the campus of UC San Diego and to researchers across the globe. As no two samples are alike, complex samples typically require innovative approaches for their analysis. Comprehensive compositional analysis of complex and environmental samples can be implemented through the following state-of-the-art analytical systems within the ECAL facility:
- Ultra-high resolution hybrid linear ion trap-Orbitrap mass spectrometer
- Gas chromatograph coupled to a triple quadrupole MS
- 2-D and 1-D ultra-performance liquid chromatography systems with UV/Vis and electrochemical detection
- Nano-liquid chromatography systems
- Inductively-coupled plasma quadrupole mass spectrometer
- Ion chromatograph with conductivity detector
All of the UPLC systems are capable of being hyphenated to the LIT-Orbitrap MS system. Furthermore, other hyphenated and unique techniques that are available (but are not limited to) include: UPLC-Orbitrap-ICPMS, IC-ICPMS, single-particle-ICP-MS (spICP-MS), SPME-GC-MS, and thermal desorption/pyrolysis-GC-MS. For inquiries about these unique approaches and the possibility for others not listed here, please contact the ECAL staff. Routine mass spectrometry applications include organic, organometallic, inorganic and elemental analyses. For organic and organometallic compounds, available measurements include high accuracy molecular weight determination, structural characterization through hyphenated MS techniques (including CID, HCD and ETD), as well as quantitative analysis through both GC-MS and UPLC-MS. For inorganic and elemental analysis, available measurements include quantitation of inorganic ions and individual elements, speciation of individual elements (e.g., simultaneous quantitation of Fe2+ and Fe3+), as well as the characterization of metallic nanoparticles.
Contact Us
ECAL Director:
Neal Arakawa
narakawa@ucsd.edu
(858) 822-3330
Mailing Address:
Environmental and Complex Analysis Laboratory
University of California, San Diego
Urey Hall 1204, MC 0314
9500 Gilman Dr.
La Jolla, CA 92093-0314
Instruments, Tools, & Resources
Instruments:
*This instrument is available for sample submission only*

The GC-TSQ MS system has the TriPlus autosampler system, which provides a wide range of sample introduction capabilities. These include both liquid and gas-phase injection by syringe as well as solid-phase microextraction (SPME). For SPME, the conditioning, sampling and desorption steps can be automated.






Tools & Resources:
What is mass spectrometry?
Mass spectrometry is simply the survey and measurement of individual ions by separating them by their mass. Through such efforts, mass spectrometry is a valuable and state-of-the-art tool for characterizing the chemical composition of a wide range of samples.
Mass Spectrometry in the ECAL Facility
Due to its unequaled speed, sensitivity and versatility, mass spectrometry is a key resource that is integrated into nearly all of the analytical platforms in the ECAL facility. The first key step in mass spectrometry is the conversion of neutral atoms or molecules into ions. To allow for a range of applications, ECAL offers a diverse catalog of ionization source platforms that accommodate a range of analyte and sample types.
Currently the ECAL facility houses an ultra-high resolution Orbitrap Elite MS system. This system is capable of accurate mass measurement within ±1 ppm (mass measurement error in terms of parts-per-million). Such high accuracy in the measured mass is high beneficial in assigning empirical formulas to unknown molecules, a key step in compound identification. Furthermore, the Orbitrap Elite system is a hybrid mass analyzer system, combining a linear ion trap (LIT) and an Orbitrap mass analyzers. The dual analyzer system allows for MS/MS and MSn techniques for structural elucidation, which coupled to the high accuracy mass measurement of the Orbitrap provides an unparalleled method for compound identification.
What is Liquid and Gas Chromatography?
Chromatography is, fundamentally, a powerful technique for separating the chemical constituents within a complex sample. Such separation can be performed by inducing interactions between neutral molecules (i.e., interaction chromatography), interactions between charged species (e.g., ion-exchange chromatography; see section on ion chromatography below), or based on molecule size (e.g., size-exclusion chromatography). High-performance liquid chromatography (HPLC) performs the separation with the constituents in the liquid phase while gas chromatography (GC) does so in the gas phase. The separation columns are specifically designed to either separate a wide range of analytes or for a specific class of analytes. Separations can be done for neutral organic molecules (e.g., liquid and gas chromatography) or ions (e.g., ion exchange chromatography).
LC and GC in the ECAL Facility
ECAL provides a range of GC and HPLC platforms capable of separating neutral organic molecules as well as organic and inorganic ions. In addition, all separation platforms are coupled to various detectors, with the most common being a mass spectrometer (GC-MS and HPLC-MS). By coupling chromatography-based separations to mass spectrometry detection, the molecular and elemental compositions of complex samples and mixtures can be easily characterized. In addition to MS detection, spectroscopic (UV/Vis) and electrochemical detection platforms are also available.
The ECAL facility houses a catalog of HPLC columns, each tailored for specific applications. This includes reverse-phase, normal-phase, HILIC, size-exclusion, and ligand-exchange columns. Most of the columns are used in applications utilizing MS and/or UV/Vis detection. The ligand-exchange columns are typically used with electrochemical detection for separating and detecting saccharides and other electroactive species in aqueous systems.
GC analyses in ECAL can be performed with either MS detection or a flame ionization detector (FID). GC-MS is performed with a triple-quadrupole detection system, which is capable of performing MS/MS techniques that allows for molecular structure determinations. Multiple sample introduction techniques are currently available, including automated liquid and headspace injection as well as automated and manual solid-phase microextraction (SPME). Analyses with the triple-quadrupole MS system can also be performed without the GC, using thermal desorption or flash pyrolysis sample introduction techniques.
A variety of GC columns are available in the ECAL facility, fitting applications ranging from non-polar to polar analytes.
LC and GC eLearning
HPLC Method Translator
GC Method Translator
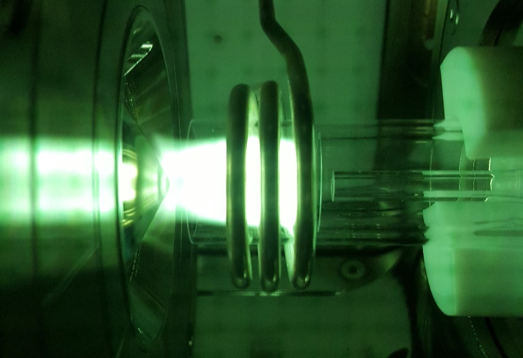
What is ICP-MS?
Inductively-coupled plasma mass spectrometry (ICP-MS) is a method of mass spectrometry capable of detecting most metals and metalloids across the periodic table. ICP-MS is a highly sensitive technique, providing the ability to detect certain metals (those which possess low background levels) down to the parts-per-quadrillion (e.g., pg/L) level. The ICP-MS uses a high-temperature source (consisting of an argon plasma at ~10,000 K) which converts the sample into individual charged atoms that are separated by the mass analyzer inside the mass spectrometer.
ICP-MS in the ECAL Facility
ICP-MS platforms in the ECAL facility offer a wide range of configurations, each tailored for specific applications and the data desired. Various sample introduction techniques are available to accommodate samples of low or high volume, including infusion by peristaltic or syringe pump as well as self-aspiration with micro-flow nebulizers. In addition to simple metal quantification in aqueous samples, the ICP-MS can be used as a detector following separation of metals by ion-exchange chromatography (IC-ICP-MS). IC-ICP-MS can be used to quantity metals with different oxidation states (e.g., measuring the amount of both Fe2+ and Fe3+). The ICP-MS system can also be configured for the measurement of individual nanoparticles (i.e., single-particle ICP-MS or spICP-MS). This novel method provides the ability to detect and quantify the metal content of individual nanoparticles in a solution and convert the quantity to a nanoparticle size.
Links to Suggested Reagents, Standards and Labware for ICP-MS Analysis
High-Purity Acids for ICP-MS
ICP-MS Standards
Volumetric Flasks
ICP Operations
Sample Preparation for ICP-MS
Guide to Trace Analysis with ICP-MS
Interactive Periodic Table of Elements for ICP-MS Analysis
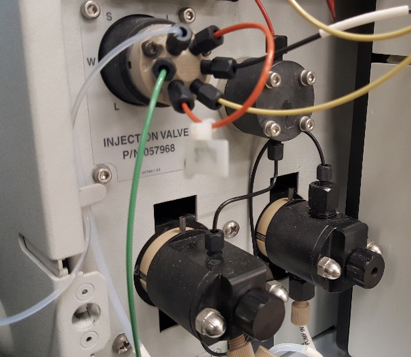
What is Ion Chromatography?
Ion chromatography (IC) is the separation of ions and polar molecules by interactions of the ions and molecules with an ion exchanger (typically a charged functional group attached to a polymeric support). Coupling IC to a conductivity detector (IC-CD) provides the ability to quantify ions down to the parts-per-billion level.
IC in the ECAL Facility
The IC-CD system in ECAL can separate and quantifying organic and inorganic cations and anions. ECAL houses a catalog of IC columns that are designed for a broad range of ions or for specific applications (e.g., separation of sulfate and sulfite). The IC system can, alternatively, be coupled to the ICP-MS (IC-ICP-MS) for separating and quantifying metals of different oxidation states.
Links to Other Tutorial and Learning Resources
Practical Guide to IC
Theory of IC
Rates of Use
| Analysis Description | Sample Introduction Method | Billing Unit | UCSDa | Non-UCSDa |
| Accurate mass determination (HRMS) | Direct Infusion | Per Hour | $80 | $116.00 |
| UPLC | Per Hourb | $85 | $123.25 | |
| Structural elucidation (MSn)c | Direct Infusion | Per Hour | $80 | $116.00 |
| UPLC | Per Hour | $85 | $123.25 | |
| Quantitative analysis | UPLC | Per Hourd | $85 | $123.25 |
a The analysis of submitted samples are subject to an additional fee of $100/hr ($145/hr for non-UCSD) to cover labor costs for ECAL personnel performing the analysis.
b For analyses using UPLC separations, the total analysis time will be used in determining the number of units charged.
c Includes CID, HCD and ETD fragmentation methods.
d Charges for quantitative analysis will include the analysis of blanks, calibration solutions and quality control solutions.
| Analysis Description | Sample Introduction Method | Billing Unit | UCSDa | Non-UCSDa |
| Quantitative analysisb,c | UPLC | Per Hourc | $50d | $72.50d |
a The analysis of submitted samples are subject to an additional fee of $100/hr ($145/hr for non-UCSD) to cover labor costs for ECAL personnel performing the analysis.
b For analyses using UPLC separations, the total analysis time will be used in determining the number of units charged.
c Charges for quantitative analysis will include the analysis of blanks, calibration solutions and quality control solutions.
d Both UPLC systems can be hyphenated with the Orbitrap Elite. For such applications the fee schedule for “Quantitative Analysis” with “UPLC” for the Orbitrap MS will be used.
| Analysis Description | Sample Introduction Method | Billing Unit | UCSDa | Non-UCSDa |
| Structural elucidation (MSn)c | GC | Per Hour | $55 | $79.75 |
| TD-Pyb | Per Hour | $55 | $79.75 | |
| Quantitative analysis | GC | Per Hourd | $55 | $79.75 |
| Qualitative analysis | Per Hour | $55 | $79.75 | |
| SPME-GCd | GC | Per Hour | $55 | $79.75 |
a The analysis of submitted samples are subject to an additional fee of $100/hr ($145/hr for non-UCSD) to cover labor costs for ECAL personnel performing the analysis.
b TD-Py indicates the use of the direct insertion probe for introducing a solid or liquid sample into the MS by either thermal desorption or flash pyrolysis.
c Charges for quantitative analysis will include the analysis of blanks, calibration solutions and quality control solutions.
d For SPME analyses, fiber conditioning times will be added to the number of billed units and billed at half the hourly rate for GC-MS (i.e., $25/hr or $36.25/hr).
| Analysis Description | Sample Introduction Method | Billing Unit | UCSDa | Non-UCSDa |
| Quantitative analysis | Infusion | Per Sampleb | $20 | $33 |
| IC | Per Sampleb | $20 | $33 | |
| Single-particle analysis | Infusion | Per Sampleb | $20 | $33 |
a The analysis of submitted samples are subject to an additional fee of $100/hr ($145/hr for non-UCSD) to cover labor costs for ECAL personnel performing the analysis.
b Charges for quantitative analysis will include the analysis of blanks, calibration solutions and quality control solutions.
Sample Submission Guidelines
- All samples must be supplied in clearly labelled and closed plastic containers. Labels must include the last name of the submitter, last name of submitter’s supervising PI and the identifying sample label listed on the Request for Sample Analysis by ICP-MS form.
- For quantitative analyses, please submit the following in addition to your samples:
- Calibration solutions (five separation solutions prepared by serial dilution) that contain the target elements in the following concentrations: 1000 ppb, 100 ppb, 10 ppb, 1 ppb and 0.1 ppb
- Blank solutions (ultrapure water with the same mineral acid used in the calibration solutions and the samples)
- All sample containers should be cleaned beforehand by soaking in 2% HNO3 (aq) overnight, then rinsed with ultrapure water (MilliQ preferred) and then dried in a clean air fume hood.
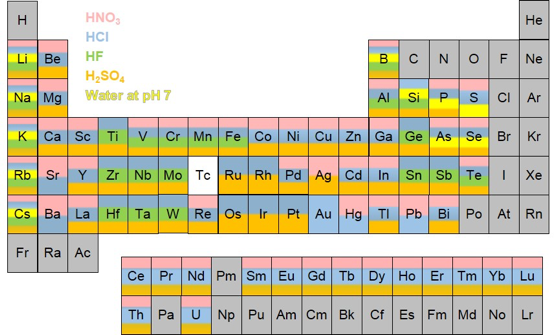
- All samples need to be submitted as either a liquid that can be analyzed by ICP-MS directly (e.g., water samples) or as dissolved samples diluted in weak mineral acid(s) (see the matric compatibility periodic table below for the appropriate acids).
- Please use trace-metal grade mineral acids for all samples and calibration solutions.
- Please ensure that the same matrix (i.e., mineral acid) as used in the sample is used in the calibration solutions and blank solutions.
- For all samples, please make note of the sample weights and dilution factors so that the final concentrations of the targeted elements in your samples can be calculated.
- It is vitally important that the submitted samples are solutions that DO NOT contain any dissolved solids (either original non-dissolved sample or salt precipitates). Samples should be filtered (at least 0.45 micron) and acidified as soon as they are collected to ensure metals are retained in solution.
- The preferred concentration of elements for analysis in solution is 10-100 ppb (parts per billion by weight; e.g., ng/g) for trace elements or 1 ppm (part per million by weight; e.g., µg/g) for major elements (i.e., Si, Ti, Al, Fe, Mg, Mn, Ca, Na, K, P).
- Sample consumption during analysis is typically between 5-10 mL. To ensure sufficient amount of sample is available, please submit 10 mL samples.
- Samples are analyzed as soon as possible upon receipt. If a more urgent timeline is needed please contact the ECAL facility staff.
- For each submitted sample and blank solutions, three individual data points (absolute intensity) are recorded and averaged. The averaged intensities are used in conjunction with the linear regression data from the calibration curves to calculate the concentration of the element in solution.
- Users will be contacted by email once samples have been analyzed. All results will be submitted by email via PDF (file titled “ECAL_ICPMS_(Last Name)-(PI Name)_Date”).
- Within the results report, all pertinent instrumental conditions, calibration curves and calculated concentrations in samples are provided.
- Both electronic and paper copies of the results are archived by ECAL staff. If needed, please contact the ECAL staff for a copy of the results.
- After analysis, all samples are refrigerated at 0-4 °C for a maximum of one month. Please ensure to retrieve your samples as soon as possible. If you require alternative sample storage conditions, please let the ECAL staff know and ensure to include such information in the sample submission form.
- Concentration of the target analyte should be around 100 µM in solution.
- Please submit the sample free of salts (e.g., Na, Mg, Ca, etc.), buffers, strong acids/bases (including TFA), and surfactants (e.g., SDS).
- Samples should be composed of LC/MS friendly solvents (e.g., water, acetonitrile, methanol, etc.).
- Use only LC/MS grade solvents when preparing your samples.
- The default ionization for LIT-Orbitrap analysis is heated electrospray (HESI). Atmospheric-pressure chemical ionization (APCI) is available upon request.
- In addition to MS detection, all UPLC analyses include parallel detection by UV/Vis adsorption spectroscopy (used to ensure proper UPLC performance in case of issues with MS detection).
- For either direct infusion (DI-MS) or UPLC-MS analysis, please inform the ECAL staff (and include the information in the sample submission form) if you require specifics for any of the following:
- mobile phase composition (including buffers),
- mobile phase program (isocratic or gradient),
- ionization parameters (including ionization polarity)
- wavelengths for UV/Vis detection.
- Please avoid using DMSO, DCM or DMF in the samples.
- All samples should be filtered prior to submission.
- Samples are analyzed as soon as possible upon receipt. If a more urgent timeline is needed, please contact the ECAL facility staff.
- Users will be contacted by email once samples have been analyzed. All results will be submitted by email via PDF (file titled “ECAL_Orbitrap_(Last Name)-(PI Name)_Date”).
- Within the results report, the following is provided:
- all pertinent instrumental conditions
- acquired MS spectra
- lists of accurate masses of target analyte
- calibration curves (if quantitative analysis is requested)
- calculated concentrations in samples (if quantitative analysis is requested)
- Both electronic and paper copies of the results are archived by ECAL staff. If needed, please contact the ECAL staff for a copy of the results.
- After analysis, all samples are refrigerated at 0-4 °C for a maximum of one month. Please ensure to retrieve your samples as soon as possible. If you require alternative sample storage conditions, please let the ECAL staff know and ensure to include such information in the sample submission form.
- Samples should be composed of GC-MS friendly solvents (e.g., dichloromethane, hexane, methanol, etc.).
- Please avoid samples containing large amounts of water.
- Use only LC/MS grade solvents when preparing your samples.
- The default ionization for GC-MS analysis is electron ionization (EI). Chemical ionization (using methane as the reagent gas) is available upon request.
- Samples are analyzed as soon as possible upon receipt. If a more urgent timeline is needed, please contact the ECAL facility staff.
- Users will be contacted by email once samples have been analyzed. All results will be submitted by email via PDF (file titled “ECAL_Orbitrap_(Last Name)-(PI Name)_Date”).
- Within the results report, the following is provided:
- all pertinent instrumental conditions
- acquired GC-MS chromatograms and spectra (upon request)
- calibration curves (if quantitative analysis is requested)
- calculated concentrations in samples (if quantitative analysis is requested)
- Both electronic and paper copies of the results are archived by ECAL staff. If needed, please contact the ECAL staff for a copy of the results.
- After analysis, all samples are refrigerated at 0-4 °C for a maximum of one month. Please ensure to retrieve your samples as soon as possible. If you require alternative sample storage conditions, please let the ECAL staff know and ensure to include such information in the sample submission form.
- Samples should either be in solid form at submission or, if in liquid form, be dissolved in a highly volatile solvent (e.g., dichloromethane, hexane, etc.)
- Please avoid samples containing large amounts of water.
- Use only LC/MS grade solvents when preparing your samples.
- The default ionization for GC-MS analysis is electron ionization (EI). Chemical ionization (using methane as the reagent gas) is available upon request.
- Samples are analyzed as soon as possible upon receipt. If a more urgent timeline is needed, please contact the ECAL facility staff.
- Users will be contacted by email once samples have been analyzed. All results will be submitted by email via PDF (file titled “ECAL_Orbitrap_(Last Name)-(PI Name)_Date”).
- Within the results report, the following is provided:
- all pertinent instrumental conditions
- acquired thermograms and
- MS spectra (upon request)
- calibration curves (if quantitative analysis is requested)
- calculated concentrations in samples (if quantitative analysis is requested)
- Both electronic and paper copies of the results are archived by ECAL staff. If needed, please contact the ECAL staff for a copy of the results.
- After analysis, all samples are refrigerated at 0-4 °C for a maximum of one month. Please ensure to retrieve your samples as soon as possible. If you require alternative sample storage conditions, please let the ECAL staff know and ensure to include such information in the sample submission form.