While our colleagues are busy with the intensive campaign measurements at the wave flume at SIO, we spread the excitement for our CAICE research at the University of Utah. Before a quite interested audience of undergraduate, graduate, postdocs, and faculty, we described our recent efforts in modeling water and ice based on “first principles” approaches with specific focus on the link between structural, thermodynamic and dynamical properties, spectroscopic signatures and reactivity. Our computer-based simulations provide new molecular-level insights into the dynamics of the hydrogen bond network in liquid water, which can be directly related to measurements of ultrafast infrared spectra. Going down in temperature, our simulations also show the importance of the quasi-liquid layer on top of ice surfaces below the melting point for understanding heterogeneous reactions that take place on ice particles in the atmosphere. A nice way of complementing the intensive studies that are going on at the Hydraulics Lab with cutting edge theoretical and computational tools.
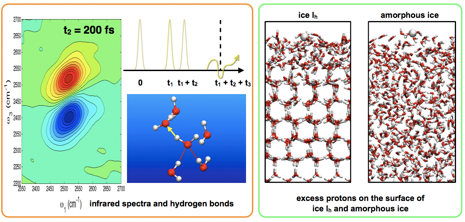
Snapshots from molecular dynamics simulations. Left: Calculated nonlinear infrared spectra in liquid water related to the dynamics of the underlying hydrogen bond network. Right: Excess protons resulting from acid dissociation on ice Ih and amorphous ice below the ice melting temperature. The presence of a thin quasi-liquid layer directly affects proton mobility and the reactivity of the two surfaces.
Stay tuned for new developments!
Francesco Paesani, Department of Chemistry and Biochemistry, UC San Diego

